- Please choose product options by visiting INDICAID® COVID-19 Antigen At-Home Test.
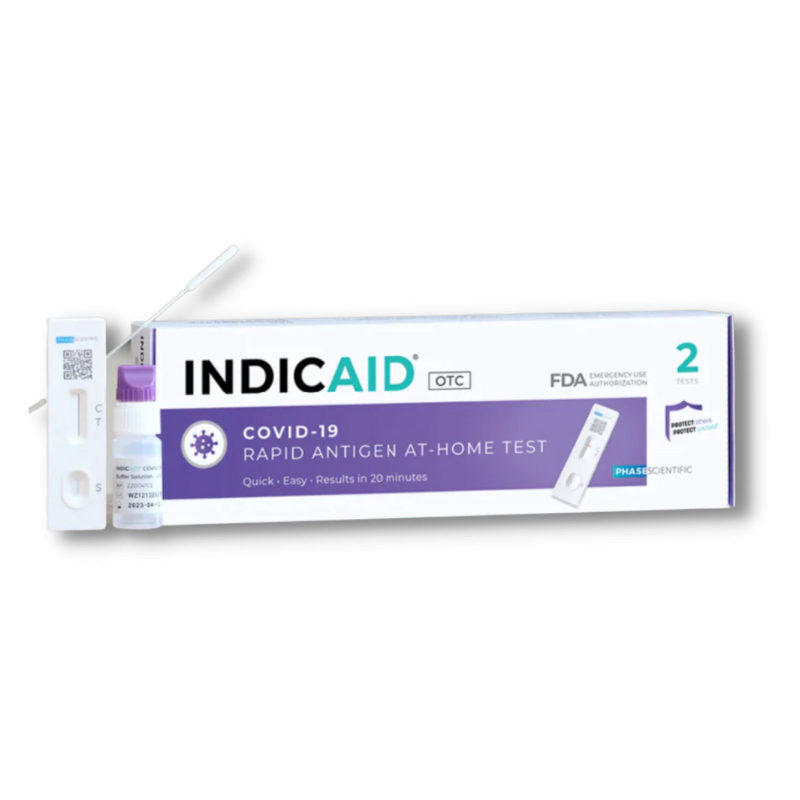
INDICAID® COVID-19 Antigen At-Home Test
In stock
INDICAID® COVID-19 Rapid Antigen Test is easy to use, allowing you to self-test anytime and anywhere to detect the presence of the SARS-CoV-2 virus. Now including Test-to-Treat powered by Dr. B—virtual, affordable treatment for COVID-19 infection. Learn more here.
SKU PHS40780P
INDICAID® COVID-19 Rapid Antigen Test is easy to use, allowing you to self-test anytime and anywhere to detect the presence of the SARS-CoV-2 virus. Now including Test-to-Treat powered by Dr. B—virtual, affordable treatment for COVID-19 infection. Learn more here.
SKU PHS40780P
The INDICAID® COVID-19 Rapid Antigen At-Home Test is intended for non-prescription self-use and/or as applicable an adult lay user testing another person 2 years of age or older. The INDICAID® COVID-19 Rapid Antigen At-Home Test is only for use under the Food and Drug Administration’s Emergency Use Authorization.
Due to FDA guidelines and supply chain demands all orders for this product are non-cancellable and non-returnable.
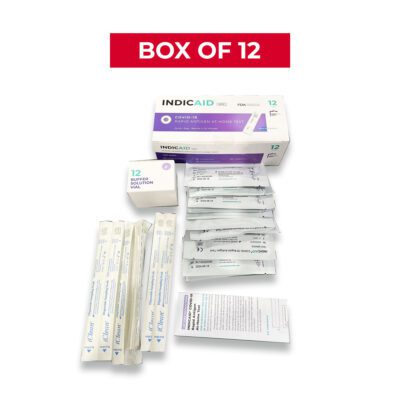
Kit Contents
- Two (2) test devices
- Two (2) shallow nasal swabs
- Two (2) buffer solutions vials with caps
- Instructions for use
Performance Measures
- 81.7% Sensitivity
- 99.4% Specificity
Features
- NEW: INDICAID® Test-to-Treat with Dr. B (learn more)
- FDA Emergency Use Authorization
- HSA/FSA Eligible
- Detects all known COVID-19 Variants
- Suitable for 2+ years old
- For symptomatic & asymptomatic individuals
- Easy to use and non-invasive
- Accurate results in 20 minutes
360 Health Services Acceptable Product Dating
We will ship product with expiration dating >= 30 days from order date.
Disclaimer
This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
| Weight | N/A |
|---|---|
| Pack Size: | Case – 126 Kits (2pk), Kit – 2 Tests |
You May Also Like
INDICAID™ COVID-19 Rapid Antigen At-Home Test (Kit/12)
In stock