Status™ COVID-19/Flu A&B Test
In stock
SKU LSN33225P
SKU LSN33225P
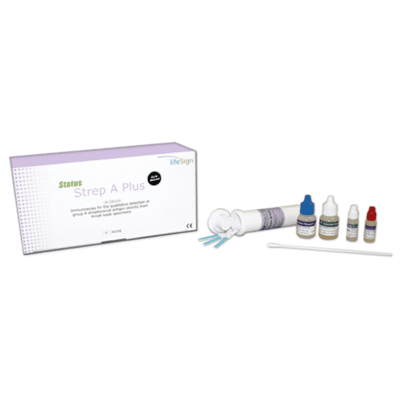
Status™ COVID-19/Flu A&B Antigen Test allows effective screening of COVID-19 and Influenza A&B with a single swab specimen collection.
Box Contents
- Twenty-five (25) Status COVID-19/ Flu A&B test devices
- Twenty-five (25) extraction reagent capsules
- Twenty-five (25) sterile swabs
- One (1) positive control swab
- One (1) negative control swab
- One (1) package insert/instructions for us
- One (1) quick reference instructions
Performance Measures
- 93.1% Sensitivity
- 100% Specificity
Features
- Designed to detect antigens from SARS-CoV-2, influenza A, and /or influenza B
- Flocked nasopharyngeal swab for superior specimen collection and patient comfort
- Visual results available in 15 minutes
- FDA Emergency Use Authorization (EUA)
Disclaimer
In the USA, this product has not been FDA cleared or approved; but has been authorized by the FDA under an Emergency Use Authorization (EUA). This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. §360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.
Due to FDA guidelines and supply chain demands, all orders for this product are non-cancellable and non-returnable.
This item is not for resale and customers must provide a valid CLIA license number associated with shipping address in order to purchase it.
| Weight | N/A |
|---|---|
| UOM | Case/1 – 375 tests, Kit/1 – 25 tests |